Explain the Difference Between Emission and Absorption Spectra
To overcome this problem. Towards Quantum Mechanical Model of Atom.

Explain The Difference Between Emission And Absorpation Spectra
In Rutherfords experiment he bombarded high energy streams of α-particles on a thin gold foil of 100 nm thickness.

. 2A shows the absorption spectra of these TPP-based luminogens in THF. Switching between filter cubes enables imag-ing of several fluorophores in a single sample. The basic computer knowledge practice problem section will get you the required practice and experience.
DTPP DTPP-Ph and DTPP-T have similar absorption profiles with peaks at 353 354 and 385 nm respectively while those of DTPP-BT DTPP-TO and DTPP-TBTT exhibit two absorption peaks with longer wavelengths at 403 426 and 508 nm respectively. It has a specific continuous spectrum of wavelengths inversely related to intensity that depend only on the bodys temperature which is assumed for the sake of. Black represents the pristine state dark.
He conducted the experiment to study the. B c The normalized OK-edge spectra and the spectra difference between two designated points in the electrochemical curve for LR b and LSLR c. Basic computer knowledge is as important to a banker as a paper is to a publisher.
This basic computer knowledge section covers many important questions for IBPS PO SBI and RBI like exams and other similar tests. However even a slight difference in alignment of the filter cubes can result in a misalignment of the images produced by the different fluorescence channels. The functional groups influence the conjugated systems causing the absorption peaks to appear at longer wavelengths than the peak wavelength of benzene.
Rutherford Atomic Model Experiment. Black-body radiation is the thermal electromagnetic radiation within or surrounding a body in thermodynamic equilibrium with its environment emitted by a black body an idealized opaque non-reflective body. The top figure shows the absorption spectra of benzene phenol which consists of a hydroxyl group bonded to a benzene ring and p-nitrophenol which consists of a hydroxyl group and a nitro group bonded to a benzene ring.
E ZPL is the zero-phonon line energy which is the energy difference between the minima of the excited- and ground-state curves plus the. In atomic physics the Bohr model or RutherfordBohr model presented by Niels Bohr and Ernest Rutherford in 1913 is a system consisting of a small dense nucleus surrounded by orbiting electronssimilar to the structure of the Solar System but with attraction provided by electrostatic forces in place of gravityIt came after the solar system Joseph Larmor model. Match the excitation and emission spectra of a number of fluorophores.
This difference could be ascribed to ICT. Emission and Absorption Spectra. The streams of α-particles were directed from a radioactive source.

What Is The Difference Between Emission And Absorption Spectrum In Tabular Form Plzzzz Chemistry Structure Of Atom 13435451 Meritnation Com

Emission Spectrum Vs Absorption Spectrum Astronomy Lessons Emissions Spectrum
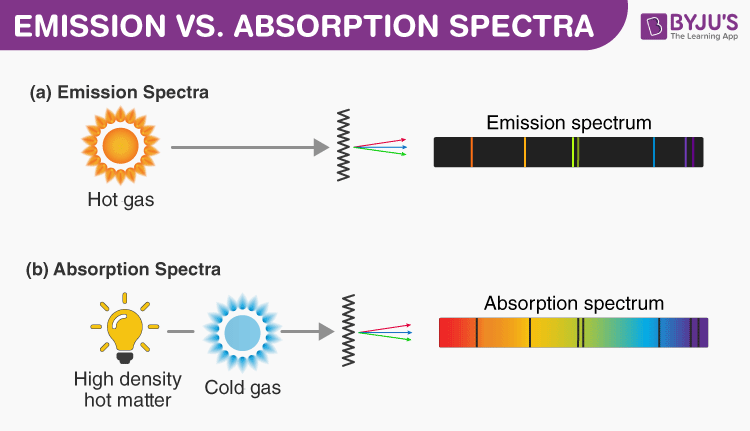
Difference Between Emission And Absorption Spectra Comparison Chart
Difference Between Absorption And Emission Spectra Definition Characteristics Comparison
0 Response to "Explain the Difference Between Emission and Absorption Spectra"
Post a Comment